
Help Ibrahim battle SPG50
Donation protected
I’m Hasnain, Ibrahim’s father. Every morning, I wake up with a sinking heart, knowing my son’s future is slipping away.
The dreams I once held, watching him run through the park, hearing his laughter as he chased butterflies, and seeing him grow into a strong, independent man have been replaced with something I never imagined.
Instead of milestones, there are setbacks. Instead of certainty, there is fear. Instead of hope, there is the crushing weight of the unknown.

Ibrahim was born on March 19, 2022 — a perfect, healthy baby with a bright smile and eyes that sparkled with joy. But as the months passed, we noticed he wasn’t reaching developmental milestones.
We consulted doctors, but for a long time, there were no answers. We told ourselves he was just developing at his own pace. Then, he began having myoclonic seizures — small, involuntary jerks that struck throughout the day. Medication helped control them, but it came at a cost: it further hindered his development.
Shortly after his second birthday, Ibrahim had a major seizure and was hospitalized.
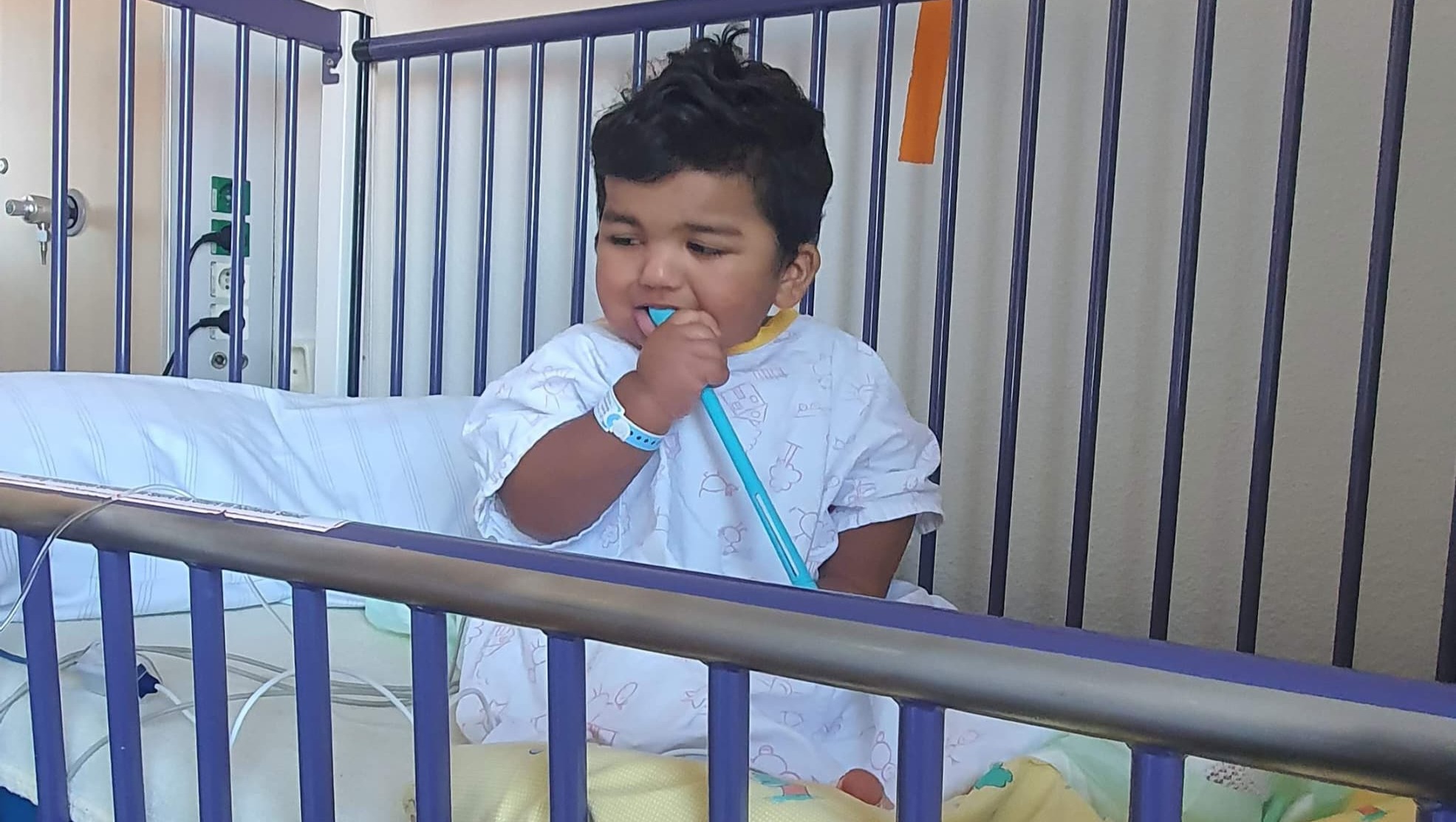
After countless visits, tests, and consultations, we received a diagnosis we never expected.
Ibrahim has SPG50 — an ultra-rare neurological disease affecting only about 80 people worldwide.
The doctor’s words shattered us: severe developmental delay, nonverbal, spastic paraplegia, confined to a wheelchair by age 10, complete paralysis afterward. A future no parent should ever have to imagine.
SPG50 is caused by the absence of a single, vital protein. Without it, the nervous system degenerates progressively taking away the ability to walk, use hands, speak, and eventually, to think clearly.
Because SPG50 is so rare, no pharmaceutical company has invested in a cure — it’s not profitable. But one father refused to accept that.
Terry, whose son also has SPG50, founded Elpida Therapeutics and helped raise over $3 million to develop a gene therapy. His son has already received the treatment. But to continue manufacturing doses for other children, more funding is urgently needed.
Elpida has now entered Phase III clinical trials:
These trials are entirely crowdfunded.
We are in continuous contact with Terry and his team. Ibrahim has been listed as a possible candidate for the clinical trial at Boston Children’s Hospital in the USA but there are no guarantees that he will be a part of it becuase of funding issues.
The current U.S. government has halted funding for research projects and this has put the clinical trial in an uncertain situation.
The cost to manufacture just 8 doses of this life-saving medicine is $2.8 million. The treatment for one child costs around $250000 an amount far beyond our reach. But with collective effort, we can make the impossible, possible.
Ibrahim is now 3 years old. He cannot walk or speak. His cognitive abilities are also affected badly, and spasticity in his legs is increasing. Every day, we watch the clock ticking on his chance for recovery.

Our only hope is this gene therapy — before it’s too late. He’s still young. The window to stop the damage is closing, but it’s not gone.
We refuse to give up. And with your help, we won’t have to.
We are not alone. Other families are fighting the same battle:
@hilfe_fuer_noemi: https://www.instagram.com/hilfe_fuer_noemi/
Dua - https://www.itv.com/news/calendar/2024-09-03/250000-to-save-girl-with-illness-affecting-only-80-worldwide
Every donation, every share, every voice raised brings us one step closer to giving Ibrahim — and children like him — a fighting chance.
Organiser
Hasnain Khalid
Organiser
Kelsterbach, Hessen